+420 241 063 352
Bioinova
A Prague-based biotech company dedicated to developing cutting-edge cell therapies and innovative medical devices.
Pioneering Cell Therapy & Diagnostics
Bioinova is a Prague-based biotechnology company driving innovation in advanced cell therapies and molecular diagnostics. Founded in 2008 as a spin-off of the Institute of Experimental Medicine of the Czech Academy of Sciences, the company transforms cutting-edge research into real-world clinical solutions, with a strong track record of patents and scientific publications.
We manufacture our own stem cell-based medicinal products, test them in preclinical and clinical studies.
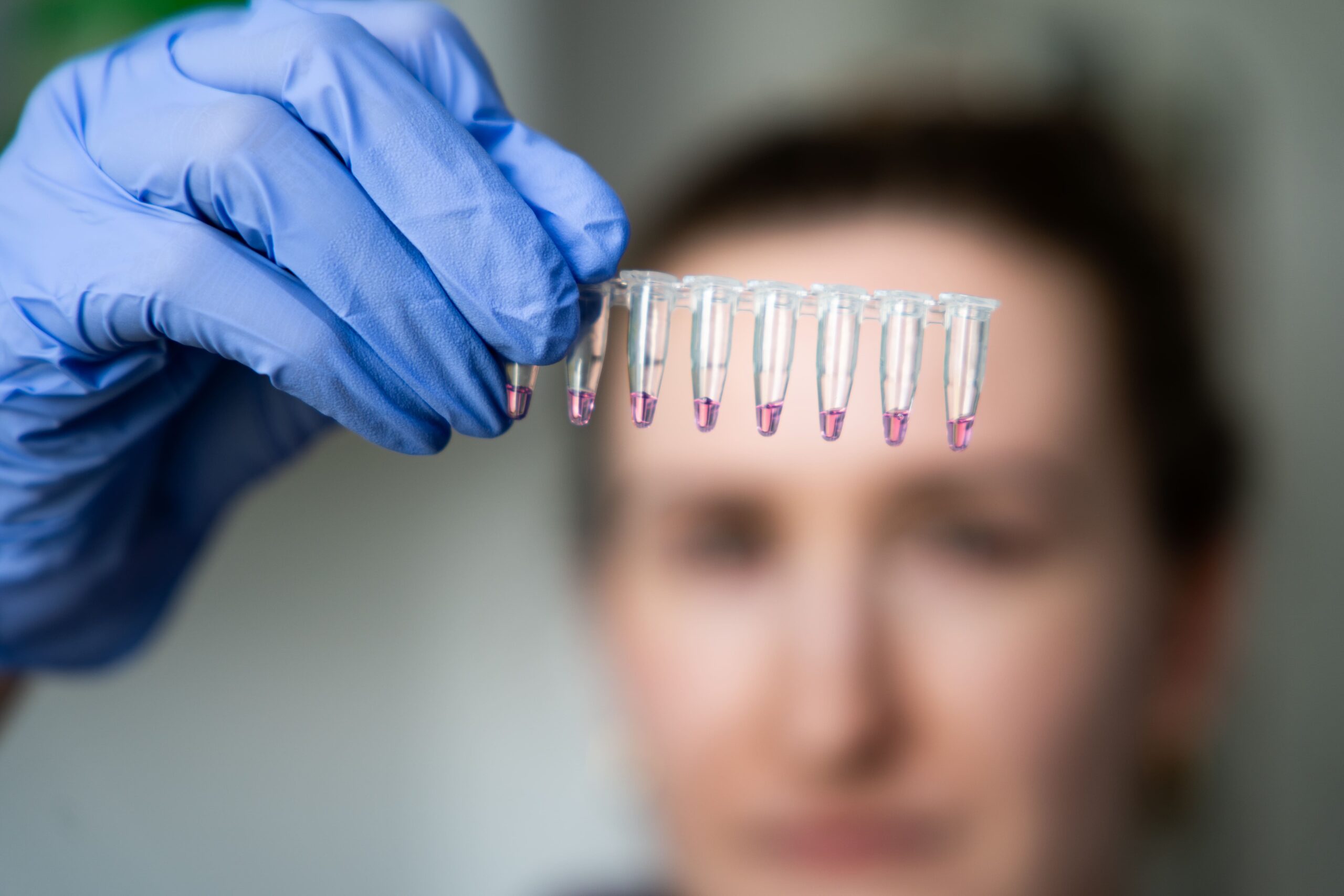
Beyond regenerative medicine, Bioinova actively addresses infection diagnostics and antimicrobial resistance. Its innovations range from PCR sampling kits that remove the need for DNA/RNA isolation, to rapid tests that distinguish viral from bacterial infections—empowering physicians with faster, more precise treatment decisions.
Through these technologies, Bioinova contributes to personalized medicine and enhances the diagnosis and treatment of diverse diseases.
Quality and compliance are ensured by comprehensive authorizations and certifications, including licenses for the manufacture of medicinal products and the operation of a tissue establishment and tissue bank. Certified under GMP, ISO 9001, and ISO 13485, Bioinova undergoes regular independent audits that ensure the highest standards of its manufacturing standards and control processes.
Products & Services
Medicinal products
(for physicians and patients)
Development of cell therapies for various fields of regenerative medicine
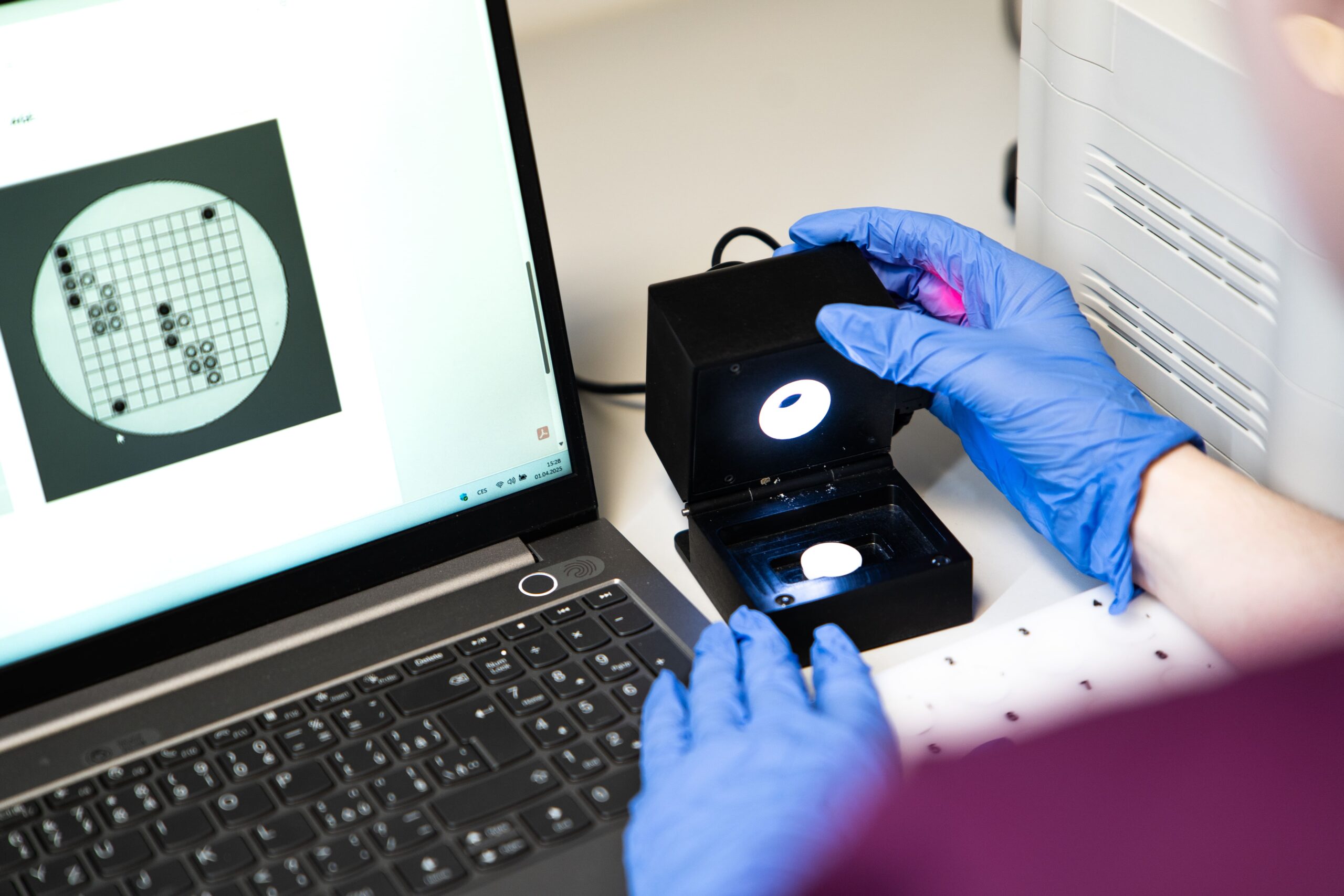
In Vitro Diagnostics
(for distributors, physicians, and diagnostic laboratories)
In vitro diagnostic medical devices for the detection of infectious diseases
Diagnostic laboratory
(for physicians and patients)
Accredited laboratory providing comprehensive services in molecular diagnostics
Tissue bank
(for parents and dental practices)
Storage of children’s dental pulp for the manufacture of stem cell-based medicinal products
Solutions for the storage and transport of stem cells
(for pharmaceutical companies and research)
Specialized formulations of advanced medicinal products and research
Meet our Experts

Peter Bauer, M.D., Ph.D.
CEO, Executive Head, Pharmacovigilance Manager
Peter completed his medical and postgraduate (Molecular Genetics, Biology and Virology program) studies at the 3rd and 2nd Medical Faculty of Charles University in Prague.
2002–2008: RIKEN Brain Science Institute, Wako-shi, Japan
2009–2011: National Institutes of Health, National Cancer Institute, Bethesda, MD, USA
2012–2016: Mayo Clinic, Department of Neuroscience, Jacksonville, FL, USA
Since 2016: CEO of Bioinova

Ivana Drahoradova
Coordinator of Production Activities, Qualified Person
Ivana completed her studies at the Faculty of Chemical Technology of Pardubice University. For more than 35 years, she has been working in quality control of immunological products manufactured by a Czech pharmaceutical company where she became a Qualified person in 1998. Ivana is now using her extensive experience to overseeing ATMPs production, cell and tissue harvesting, medical device manufacturing, and the operation of a diagnostic laboratory, ensuring compliance with legislation and regulatory requirements.

Katerina Ruzickova, Ph.D.
Clinical Research Associate
Katerina graduated from the Faculty of Science of Charles University in Prague (Biochemistry) and obtained her postdoctoral degree at the 1st Medical Faculty of Charles University in Neuroscience. As a visiting fellow, she had positions in Seattle, United States; Budapest, Hungary; and Cologne, Germany. After her studies, she worked at the Institute of Experimental Medicine of the Czech Academy of Sciences for 14 years. Since 2011, Katerina has been the Head of the Quality Control Department, since 2018, Qualified Person in Bioinova. In 2019, she also became the Responsible Person for the Tissue Establishment. She currently works as a Clinical Research Associate.

Jana Kvizova
Head of the Production Department
Jana graduated at the University of Chemistry and Technology in Prague (Biotechnology of Pharmaceuticals). Within her studies she had been focusing on targeted therapy of cancer and specifically, testing of novel photosensitive molecules in cell cultures. During her last year at the university, she started to work in Bioinova and subsequently joined the production team full-time in 2018. Her main RnD focus is the cryopreservation of stem cells.

Barbora Codlova
Head of the QC Department
Barbora graduated from the Czech University of Life Sciences Prague (Reproductive Biotechnologies). After completion of her studies, she worked in the Quality Control Department where she was responsible for quality control procedures of stem-cell-based products in veterinary medicine. She joined Bioinova Quality Control Department in 2021. Her main focus is quality control procedures (qPCR, flow cytometry) of ATMPs and custom quality control.

Michael Nemec
Head of Diagnostic Laboratory
Michael is a specialist with several years of experience in molecular diagnostics, microbiology, and genetic methods. He graduated in Biochemistry and Biotechnology, followed by General and Applied Biochemistry at the University of Chemistry and Technology in Prague. Michael has built his professional career in dynamic laboratory environments, including GENNET s.r.o. and Bioinova a.s., where he worked as a research specialist focusing on diagnostic microbiological and genetic methods. Since 2024, he has been leading the Diagnostic Laboratory.
David Vetvicka, Ph.D.
Head of the Tissue Bank
David has been with Bioinova since July 2023, initially overseeing the BioCanim project focused on the development of intratumoral immunotherapy. Before joining Bioinova, he spent many years at the First Faculty of Medicine, Charles University, where he specialized in the experimental development of cancer therapies. David has extensive experience in the biological evaluation of systems for targeted delivery of anticancer drugs and their effects on the immune system. During his career, he also worked as a researcher at renowned international institutions, including the University of Oxford, the University of Minnesota, and the Nebraska Medical Center in the USA. Beyond academia, he founded and led Sven BioLabs, a company distributing products for research laboratories, as well as a pharmaceutical start-up developing a drug against tuberculosis. At Bioinova, he is responsible for the Tissue Bank, where he can apply his experience from both academic and commercial spheres.

Hana Potockova, Ph.D.
Consultant
Hana graduated from the Faculty of Science at Masaryk University in Brno and subsequently earned a Ph.D. in Drug Safety and Quality at the Faculty of Pharmacy. Since 2014, she has held various positions in companies that produce medicinal products and medical devices, participated in international collaborations, and led several development projects. At Bioinova, she serves as a consultant in the areas of clinical trials and regulatory affairs.